
Are you ready for a revolution in pharmaceutical testing? For decades, the U.S. Food and Drug Administration (FDA) has required animal testing before human clinical trials as a prerequisite for approving new drugs to hit the market. But in December 2022, a game-changing shift occurred. President Joe Biden signed new legislation giving the FDA the power to greenlight a drug or biologic for human trials based on non-animal testing methods.
This marks a significant step forward in modernizing science and could signify the beginning of a transformative new era in drug testing. So, is this the start of a transformation of pharmaceutical testing? Only time will tell.
In this article, we’ll explore the history of animal testing and alternatives options.
The Beginning and the End of FDA Animal Testing
The debate surrounding animal testing in the drug discovery process has been ongoing for centuries. While recent laws have aimed to make animal testing more humane, the question of whether or not animals can truly replicate human reactions to drugs remains a contentious issue.
It wasn’t until the tragic mass poisoning of 1937 caused by a new product that hit the market without proper animal testing, that the “need” for animal testing was brought to light. This disaster, where over 100 people died from taking a preparation of sulfanilamide using a toxic solvent, led to the passing of the Federal Food, Drug, and Cosmetic Act in 1938, requiring safety testing of drugs on animals before they can be marketed to the public.
Fast forward 84 years, the FDA animal testing laws have undergone a significant change with the introduction of the FDA Modernization Act 2.0. Senators Rand Paul and Cory Brooker, along with Rep. Vern Buchanan, introduced the act in early 2022 and it was passed unanimously in September. The act aims to accelerate innovation and get safer, more effective drugs to market more quickly by cutting red tape that is not supported by current science. It is not a ban on animal testing, but it allows companies to use other methods of testing to get drugs approved without the use of animals.
Alternatives to Animal Testing
Over the past decade, scientists have been working tirelessly to develop alternative testing methods that are not only more humane but also provide more accurate results that can be applied to humans. From using human cells and tissues in-vitro to advanced computer-modeling techniques, and even human volunteers, these alternatives are revolutionizing the way drugs are developed and tested.
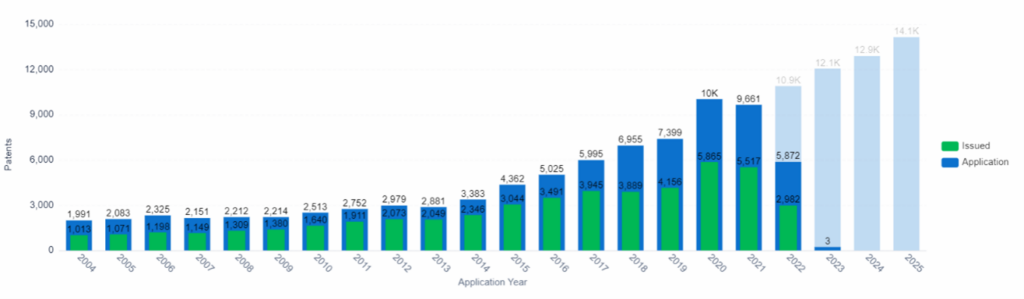
Patent Application and Issue Trend, Organ-on-chip, PatSnap Insights (Note: Data from 2022 onward is incomplete due to the 18-month filing lag).
In this section, we’ll delve into the exciting world of alternative testing methods, showcasing the cutting-edge techniques that are being used by leading drug companies to reduce their reliance on animal testing.
This article was curated using our AI-powered Discovery Product, which connects billions of patent and non-patent data points. Get the technology insights and competitive intel you need.
FDA Animal Testing Alternative #1: In Vitro Testing
In vitro pharmacology is the process of carrying out drug experiments on microorganisms and cells outside of their normal biological environment (e.g., tumor cancer cells in a petri dish). This is one of the key methods which researchers use to obtain data on the efficacy and safety of drug candidates. For the lead optimization stage of drug discovery, in vitro pharmacology can generate high levels of precise data in a timely manner, allowing the identification of the compound which will suit the condition best. Only the compounds that pass this stage will go on to pre-clinical trials.
Other examples of where in vitro pharmacology include:
- To collect data on the safety and toxicity of a compound
- To identify potential adverse effects early in the process
- Guidance for pre-clinical in vivo toxicity and safety studies, based on profile compounds.
- Predict potency and efficacy of the compound against the selected disease.
- Understand the pharmacokinetics and pharmacodynamics of the compound.
- Study the compound’s mechanism of action in more depth.
- Evaluate biosimilar activity.
The in vitro toxicology testing market is set to explode in the next few years, with projections of reaching a staggering $16.5 million by 2028 and a compound annual growth rate (CAGR) of 6.5% from 2022 to 2028. But what’s driving this growth?
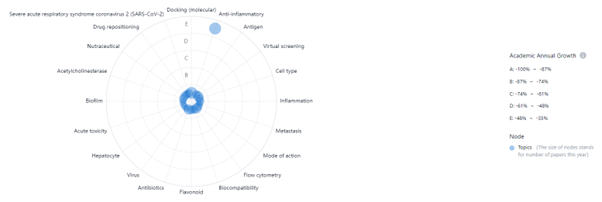
Emerging Tech, In Vitro Toxicology, PatSnap Discovery
It’s a combination of factors, from the latest technological advancements to the increasing ethical concerns surrounding animal testing. Add in the high cost of traditional animal testing and the increasing R&D spend to identify toxicity early in the drug development process, and it’s clear that in vitro toxicology testing is one viable alternative.
FDA Animal Testing Alternative #2: Miniature Organs:
Organs-on-a-chip, which are made by growing human cells on plastic scaffolds, are the most widely used “miniature” organs. These tiny structures mimic the functions of organs such as the heart, liver, kidneys, and lungs. Researchers use these structures to study how liquids flow through the organs, simulating blood flow and fluid tracking through tissues. For example, the liver plays a key role in metabolism, so a miniature liver can be used to test the toxicity of drugs by observing damage to the cells in the system.
Donald Ingber, a bioengineer at Harvard University, developed organ chip technology and partnered with Emulate. He published a study on the success of the chips, which found that the liver chips correctly identified 87% of a variety of drugs that went on to human trials after animal studies which failed due to liver toxicity. The chips also did not falsely flag any non-toxic drugs.
The organ-on-chip market is projected to experience significant growth in the coming years. In 2021, the market was valued at $150.3 million and it’s estimated to reach $1,427.4 million by 2029, with a CAGR of 28.25% during the forecast period. This growth can be attributed to factors such as an increasing demand for organ-on-chip devices in the healthcare industry, and an increasing number of clinical trials that use these devices to study the toxicity of drugs and cosmetics, and compare results between human and animal subjects.
FDA Animal Testing Alternative #3: Computer Modeling
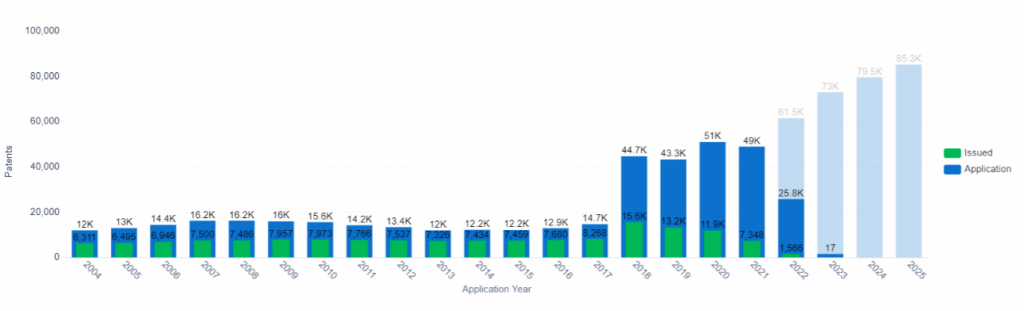
Patent Application and Issue Trend, Computer Modeling, PatSnap Insights (Note: Data from 2022 onward is incomplete due to the 18-month filing lag).
The use of data in the drug development process is increasing, with researchers utilizing computational models to analyze large amounts of data in order to predict the effects of compounds on organisms.
A team led by Hao Zhu, an associate professor of chemistry at Rutgers University, has developed an algorithm that extracts data from chemical databases and compares tested and untested compounds to identify structural similarities and potentially predict their mechanism of action. Studies, such as one from John Hopkins University, have shown that using algorithms can be more beneficial than testing on animals for predicting toxicity. For example, a 2018 study at the University of Oxford found that computer simulations outperformed animal studies with an accuracy of 89-96% in predicting the clinical risk of drug-induced arrhythmias.
The market growth
of computer-aided drug discovery is predicted to bring in $7.5 million
between 2022 to 2030, with a CAGR of 11.48%. Factors driving this growth
include chronic, unknown, and rare diseases which currently lack viable
treatments or offer limited options.
FDA Animal Testing Alternative #4: Human Volunteer Studies
Is it really ethical to use human volunteers as test subjects for drug testing? It’s debatable. However, advances in technology are maker it safer. For example, sophisticated scanning machines, such as those used to conduct brain scans, can monitor the progression and treatment of brain diseases.
As a result, these machines can also provide insight into brain function which is especially useful when studying nutrition, drug addiction, or pain levels. Once complete, the findings are compared to healthy volunteers, which offers a comprehensive understanding of how treatments affect the human body.
FDA Animal Testing Alternative #5: Micro-dosing
Another aspect of “human pre-clinical” trials, is micro-dosing. These are carefully controlled forms of human studies, which do not endanger the health of the participants. Micro-dosing involves giving human volunteers a new drug in very small doses, where it doesn’t have large physiological effects, but there is enough of the substance in the system to affect cells. These types of tests are done to eliminate nonviable drugs in the early stages, thus saving money on expensive animal testing for a compound doomed to fail. This approach has been proven safe and effective and is being adopted by many pharmaceutical companies to help streamline their drug development process.
Micro-dosing studies provide several strategic benefits, such as resolving discrepancies or inadequacies in pre-clinical data, allowing for efficient movement into human testing with limited resources, and accelerating drug development for vulnerable populations. The primary benefit of these phase 0 studies is the data on pharmacodynamics (PD) and mechanism of action (MOA) that they provide, which supports the drug development process.
FDA Animal Testing Alternative #6: Human Tissue Samples (Ex Vivo)
Ex vivo refers to the use of whole tissues to predict drug efficacy and safety. Human tissue tests are commonly used in the drug development process to understand how compounds will affect humans. Diseases such as diabetes and cancer can occur in both humans and animals, but there are differences that can lead to discrepancies between results from animal tests and those from human tests.
Human tissue samples can come from:
- Surgical residual material: In surgery, tissue can be sent for diagnostic testing and if there is any left over, the pathologist can decide if it can be donated to research.
- Transplant tissue: If there are reasons why an organ cannot be used for transplantation, then it can be used for research purposes.
- Biopsy material: This material is donated directly from patients or volunteers.
Fresh tissue samples are mainly utilized in late-stage pre-clinical testing or lead optimization to determine if cell-based or animal data applies to humans. Researchers can also use these samples to investigate if similar signaling pathways are activated and if specific biomarkers are present that indicate efficacy. Using human tissue samples for testing in later stages of development can save pharmaceutical companies significant amounts of money, as failure rates are high in these stages.
The global tissue diagnostic market size was valued at $37.8 billion in 2022 and is expected to reach $122.7 billion by 2032, with a CAGR of 12.5%. The primary driver of this growth over the next 10 years is the increasing elderly population. The United Nations World Population Ageing study predicts that the number of people over 65 will reach 1.6 billion by 2050. As the population ages, diseases such as cancer, diabetes, and cardiovascular disorders become more prevalent, which in turn increases the demand for new tissue diagnostic therapies.
Conclusion: Animal Testing May Go Extinct
With the passing of the FDA Modernization Act 2.0, pharmaceutical companies now have the option to develop therapeutics without the use of animals. Greater public awareness of which drugs are tested on animals, and which are not, will likely lead to a shift in consumer behavior. This presents a significant opportunity for further development and increased trust in alternative testing methods.
Unlock the power of AI-driven insights to stay ahead of the curve in R&D! Try our cutting-edge solution, Synapse, for free and revolutionize your life science organization’s research and development efforts today.
